Abstract

Myelofibrosis (MF) is associated with splenomegaly, cytopenias, constitutional symptoms, and bone marrow fibrosis, with limited therapeutic options. Currently only two Janus kinase inhibitors (JAKi) are approved for MF worldwide. Jaktinib, an oral novel JAKi is under investigations. We present the results from a Phase 2 open-label and multi-center study, evaluated two different regimens of Jaktinib in patients (pts) with MF.
Aims: To investigate the efficacy, safety and pharmacokinetics (PK) of Jaktinib in MF pts.
Methods: Eligibility: MF pts included primary per WHO criteria (2016) or post-essential thrombocythemia / polycythemia vera MF according to IWG-MRT criteria; DIPSS-PLUS ≥ int-2 (int-1 with symptomatic splenomegaly/hepatomegaly required a treatment). From 21 study sites, 104 pts were randomized by 1:1 ratio to Jaktinib 100mg BID or 200mg QD during the first stage, then 14 additional were enrolled to Jaktinib 100mg BID in the second stage, total 118 pts participated in the study. The primary endpoint: the proportion of pts with spleen volume reduction from baseline ≥ 35% (SVR35) at week 24 (W24), assessed by Independent Review Committee based on MRI/CT images. Secondary endpoints: at W24 compared to baseline, the proportion of pts with ≥ 50% reduction on MPN-SAF TSS, improvements in RBC transfusion and hemoglobin (Hgb), safety profiles as well as the PK characteristics, etc.
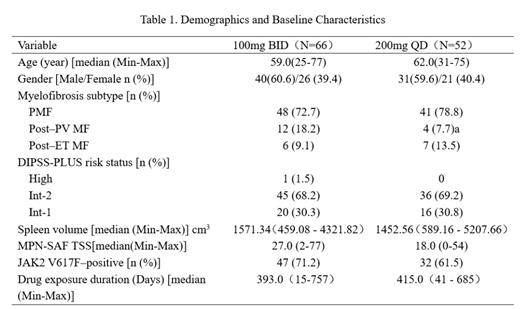
Results: 118 pts who completed treatments of 24 weeks or planned visits were summarized. Baseline characteristics were generally balanced between the two groups, and shown in Table 1.
At W24, the SVR35 were 51.5% (34/66) in the 100mg BID and 28.8% (15/52) in the 200mg QD (p=0.0151), the median duration of response has not reached yet for the 100mg BID and 11.0 months for the 200mg QD. The median time to achieve a first SVR35 was 5.5 months and 11.0months, respectively.
The proportion of pts achieved ≥50% improvement in TSS at W24 from baseline for BID and QD arms were 63.6% (42/66) and 53.8% (28/52), respectively. The mean percent of TSS change from baseline was -62.00 and -42.01, respectively. Approximately 35.6% (21 /59) of combined 59 pts whose baseline's Hgb ≤100g/L had elevated ≥20g/L at W24. Of the 6 pts who were transfusion-dependent at baseline, 2 pts became transfusion-independent after treatment. In addition, 5 (71.4%) out of 7 pts had a RBC infusion decreased ≥50%.
The most common Grade ≥3 hematological TEAEs (≥ 5%) were anemia (100mg BID 24.2%, 200mg QD 28.8%), thrombocytopenia (16.7%, 11.5%), neutropenia (3.0%, 11.5%), and leukopenia (0, 7.7%). Most common non-hematological (of any grade) TEAEs (≥10%) were upper respiratory tract infection (24.2%, 30.8%), elevated creatinine (19.7%, 30.8%), elevated ALT (24.2%, 21.2%) and elevated bilirubin (24.2%, 13.5%), predominantly in Grade 1 or 2.
A total 64 (54.2%) of the combined 118 pts had ≥ 1 dose adjustment/interruption, mostly due to thrombocytopenia (31.4%), anemia (22.0%), and neutropenia (8.5%), while 10 (10.2%) pts discontinued the treatment because of adverse events (thrombocytopenia[n=3], anemia[n=2,with 1 pts occurring pneumonia at the same time], pneumonia[n=1], tuberculosis[n=2], upper gastrointestinal bleeding[n=1], chronic kidney disease[n=1]).
Total 23 (100mg BID 12 and 200mg QD 11) pts had their blood PK samples collected per protocol schedule. After single oral administration, the median T max is 2h and the average t 1/2 is 3~4 h in both groups. The AUC 0-24 of Jaktinib and its metabolites on the first day were comparable in both groups. After multiple doses, in comparison to 200mg QD, 100mg BID had a decreased C max and an increased C trough, resulting in less fluctuation between the peak and trough concentrations, which indicated that continual inhibitions of JAK-STAT pathway may result in better clinical outcomes.
Summary/Conclusion: Jaktinib as a novel JAKi is generally well-tolerated and safe in Chinese MF pts. Significant reductions in spleen volume and constitutional symptom burden were observed in the 100mg BID, and also a sign of improvement was shown in RBC transfusion dependency and Hgb levels after Jaktinib treatment. Follow-up study is on-going and will provide long-term efficacy and safety data of Jaktinib.
Zhou: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Jiang: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Wu: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Zhuang: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Li: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Wang: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Huang: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Zhu: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Zhang: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Du: Suzhou Zelgen Biopharmaceuticals Co.,Ltd.: Honoraria. Xiang: I agree not to accept honoraria or reimbursement, including travel support, from ineligible companies for my role as a chair/moderator/presenter in the accredited portions of this activity: Honoraria. Zhang: The content I am responsible for will be free of logos or other corporate identifiers of healthcare industry companies, specifically those whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or : Honoraria. Hu: Astellas Pharma, Inc.: Research Funding. Liu: The content I am responsible for will be free of logos or other corporate identifiers of healthcare industry companies, specifically those whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or : Honoraria. Jin: The content I am responsible for will be free of logos or other corporate identifiers of healthcare industry companies, specifically those whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or : Honoraria. Sun: The content I am responsible for will be free of logos or other corporate identifiers of healthcare industry companies, specifically those whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or : Honoraria. Zhou: The content I am responsible for will be free of logos or other corporate identifiers of healthcare industry companies, specifically those whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or : Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal